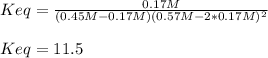Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

You know the right answer?
9. Given the following reaction:CO (g) + 2 H2(g) CH3OH (g)In an experiment, 0.45 mol of CO and 0.57...
Questions







Arts, 11.10.2019 10:10


Mathematics, 11.10.2019 10:10

Mathematics, 11.10.2019 10:10


Computers and Technology, 11.10.2019 10:10






History, 11.10.2019 10:10


Business, 11.10.2019 10:10

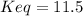
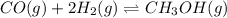
![Keq=\frac{[CH_3OH]}{[CO][H_2]^2}](/tpl/images/0694/1862/81c3a.png)
 due to the reaction extent we can write:
due to the reaction extent we can write:![Keq=\frac{x}{([CO]_0-x)([H_2]_0-2x)^2}](/tpl/images/0694/1862/e4583.png)
![[CO]_0=\frac{0.45mol}{1.00L}=0.45M\\](/tpl/images/0694/1862/3035d.png)
![[H_2]_0=\frac{0.57mol}{1.00L}=0.57M\\](/tpl/images/0694/1862/dc61d.png)
![[CO]_{eq}=\frac{0.28mol}{1.00L}=0.28M\\](/tpl/images/0694/1862/ecf3a.png)
![x=[CO]_0-[CO]_{eq}=0.45M-0.28M=0.17M](/tpl/images/0694/1862/8aaf6.png)