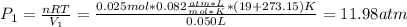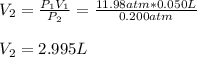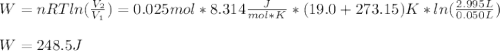A quantity of 0.0250 mol of a gas initially at 0.050 L and 19.0°C undergoes a constant-temperature expansion against a constant pressure of 0.200 atm. If the gas is allowed to expand unchecked until its pressure is equal to the external pressure, what would its final volume be before it stopped expanding, and what would be the work done by the gas?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

You know the right answer?
A quantity of 0.0250 mol of a gas initially at 0.050 L and 19.0°C undergoes a constant-temperature e...
Questions

English, 20.10.2020 20:01


Mathematics, 20.10.2020 20:01


Chemistry, 20.10.2020 20:01



History, 20.10.2020 20:01

English, 20.10.2020 20:01




Arts, 20.10.2020 20:01


Mathematics, 20.10.2020 20:01

Advanced Placement (AP), 20.10.2020 20:01




English, 20.10.2020 20:01

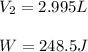
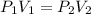
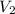 by firstly computing the initial pressure:
by firstly computing the initial pressure: