Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:30
In which direction will the following reaction go if the standard reduction potentials are 0.80 v for ag/ag+ and –0.44 v for fe/fe2+? ag+ + fe → ag + fe2+ a.)forward b.)the reaction cannot occur. c.) not enough information is given. d.) reverse
Answers: 1

Chemistry, 21.06.2019 18:00
Rutherford's experiment indicated that matter was not as uniform as it appears what part of his experimental results implied this idea
Answers: 1

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2
You know the right answer?
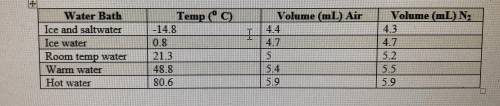
If the atmospheric pressure in the laboratory is 1.2 atm, how many moles of gas were in each syringe...
Questions




Social Studies, 14.12.2021 01:10


SAT, 14.12.2021 01:10

Mathematics, 14.12.2021 01:10


Mathematics, 14.12.2021 01:10






Mathematics, 14.12.2021 01:10

Mathematics, 14.12.2021 01:10

Mathematics, 14.12.2021 01:10

Mathematics, 14.12.2021 01:10

Engineering, 14.12.2021 01:10