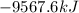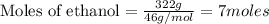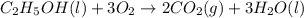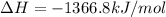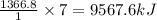Chemistry, 26.06.2020 15:01 nehaljay1883
Calculate the change in enthalpy associated with the combustion of 322 g of ethanol. C2H5OH(l)+3O2(g)⟶2CO2(g)+3H2O(l)ΔH∘ c=−1366.8kJ/mol

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Amass of 100.0 g of solute is dissolved in water so that 850. ml of a 0.7500 m solution has been prepared. what is the molar mass of the solute?
Answers: 2

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2


Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
Calculate the change in enthalpy associated with the combustion of 322 g of ethanol. C2H5OH(l)+3O2(g...
Questions

English, 26.09.2021 20:50

Mathematics, 26.09.2021 20:50

Mathematics, 26.09.2021 20:50

Mathematics, 26.09.2021 20:50

History, 26.09.2021 20:50

History, 26.09.2021 20:50

English, 26.09.2021 20:50

Geography, 26.09.2021 20:50

English, 26.09.2021 20:50



English, 26.09.2021 20:50


Mathematics, 26.09.2021 20:50

Chemistry, 26.09.2021 20:50