
Chemistry, 26.06.2020 15:01 harleycochran2ovyt3n
Phosphorus pentachloride decomposes to phosphorus trichloride at high temperatures according to the equation: PCl5(g) ⇌ PCl3(g) + Cl2(g) At 250° 13.0 g of PCl5 is added to the flask with a final solution volume of 0.500 L. If the value of Kc at this temperature is 1.80, what are the equilibrium concentrations of each gas?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
You know the right answer?
Phosphorus pentachloride decomposes to phosphorus trichloride at high temperatures according to the...
Questions

Mathematics, 08.08.2021 01:10


Social Studies, 08.08.2021 01:10

Chemistry, 08.08.2021 01:10



Mathematics, 08.08.2021 01:10

Mathematics, 08.08.2021 01:10




Mathematics, 08.08.2021 01:10



Chemistry, 08.08.2021 01:10

Mathematics, 08.08.2021 01:10

Mathematics, 08.08.2021 01:10



![[PCl_3]_{eq}=0.117M](/tpl/images/0694/2073/b10d4.png)
![[Cl_2]_{eq}=0.117M](/tpl/images/0694/2073/e8dd1.png)
![[PCl_5]_{eq}=8x10^{-3}M](/tpl/images/0694/2073/b071a.png)
![Kc=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0694/2073/c6686.png)
 due to the reaction extent:
due to the reaction extent:![Kc=\frac{x*x}{[PCl_5]_0-x}](/tpl/images/0694/2073/e60cd.png)
![[PCl_5]_0=\frac{13.0g*\frac{1mol}{208.25g} }{0.500L} =0.125M](/tpl/images/0694/2073/efcb9.png)
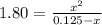
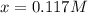
![[PCl_3]_{eq}=x=0.117M](/tpl/images/0694/2073/3ee84.png)
![[Cl_2]_{eq}=x=0.117M](/tpl/images/0694/2073/4a909.png)
![[PCl_5]_{eq}=0.125M-x=0.125M-0.117M=8x10^{-3}M](/tpl/images/0694/2073/4d0ed.png)


