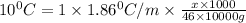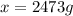Chemistry, 26.06.2020 16:01 FantasticFerret
What mass of ethanol, C2H5OH a nonelectrolyte, must be added to 10.0 L of water to give a solution that freezes at −10.0°C? Assume the density of water is 1.0 g/mL. Kf of water is 1.86°C/m.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
What mass of ethanol, C2H5OH a nonelectrolyte, must be added to 10.0 L of water to give a solution t...
Questions






Mathematics, 24.07.2019 19:20

Mathematics, 24.07.2019 19:20


History, 24.07.2019 19:20

Mathematics, 24.07.2019 19:20

Mathematics, 24.07.2019 19:20

Mathematics, 24.07.2019 19:20

English, 24.07.2019 19:20



Mathematics, 24.07.2019 19:20

Biology, 24.07.2019 19:20

Mathematics, 24.07.2019 19:20

Chemistry, 24.07.2019 19:20

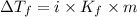
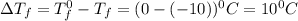 = Depression in freezing point
= Depression in freezing point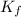 = freezing point constant for water=
= freezing point constant for water= 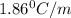
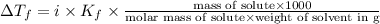
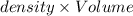
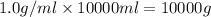 ( 1L=1000ml)
( 1L=1000ml)