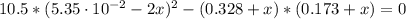Chemistry, 26.06.2020 16:01 AnxiousKid
An equilibrium mixture of the three gases in a 1.00 L flask at 350 K contains 5.35×10-2 M CH2Cl2, 0.173 M CH4 and 0.173 M CCl4. What will be the concentrations of the three gases once equilibrium has been reestablished, if 0.155 mol of CH4(g) is added to the flask?

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

You know the right answer?
An equilibrium mixture of the three gases in a 1.00 L flask at 350 K contains 5.35×10-2 M CH2Cl2, 0....
Questions


Social Studies, 06.04.2021 17:40

English, 06.04.2021 17:40



Mathematics, 06.04.2021 17:40





Mathematics, 06.04.2021 17:40

English, 06.04.2021 17:40

Mathematics, 06.04.2021 17:40

Mathematics, 06.04.2021 17:40

Mathematics, 06.04.2021 17:40

Mathematics, 06.04.2021 17:40


Mathematics, 06.04.2021 17:40

Spanish, 06.04.2021 17:40

Biology, 06.04.2021 17:40

![K = \frac{[CH_{4}][CCl_{4}]}{[CH_{2}Cl_{2}]^{2}} = \frac{0.173 M*0.173 M}{(5.35 \cdot 10^{-2} M)^{2}} = 10.5](/tpl/images/0695/0052/aba2c.png)
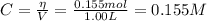
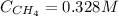
![K = \frac{[CH_{4}][CCl_{4}]}{[CH_{2}Cl_{2}]^{2}} = \frac{(0.328 + x)(0.173 + x)}{(5.35 \cdot 10^{-2} - 2x)^{2}}](/tpl/images/0695/0052/cf9a0.png)