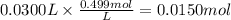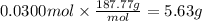Chemistry, 27.06.2020 05:01 viktoria1198zz
Aqueous solutions of copper (II) bromide and silver (1) acetate react to form solid
silver (1) bromide and aqueous copper (II) acetate according to the UNBALANCED
reaction below.
CuBr2 (aq) + AGCH3CO2 (aq)
-
AgBr (s) + Cu(CH3CO2)2 (aq)
How many grams of silver (1) bromide will form if 30.0 mL of 0.499 M copper (II)
bromide react with excess silver (1) acetate?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2



Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
Aqueous solutions of copper (II) bromide and silver (1) acetate react to form solid
silver (1) brom...
Questions

Mathematics, 02.12.2021 01:20


SAT, 02.12.2021 01:20



Mathematics, 02.12.2021 01:20




Mathematics, 02.12.2021 01:20



Social Studies, 02.12.2021 01:20


History, 02.12.2021 01:20

Mathematics, 02.12.2021 01:20

History, 02.12.2021 01:20

History, 02.12.2021 01:20


Health, 02.12.2021 01:20