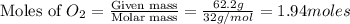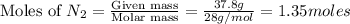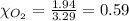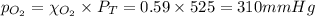Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1
You know the right answer?
A gaseous mixture of O2 and N2 contains 37.8% nitrogen by mass. What is the partial pressure of oxyg...
Questions


Mathematics, 23.03.2020 20:35


Mathematics, 23.03.2020 20:35

Mathematics, 23.03.2020 20:35

History, 23.03.2020 20:35





English, 23.03.2020 20:35




Chemistry, 23.03.2020 20:35



Mathematics, 23.03.2020 20:35


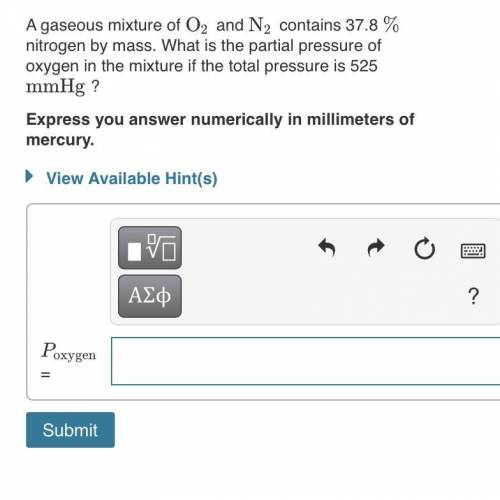
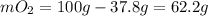
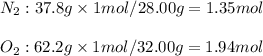
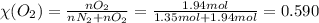
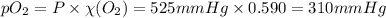
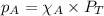
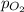 = partial pressure of
= partial pressure of 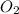 = ?
= ?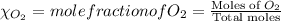
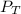 = total pressure of mixture = 525 mmHg
= total pressure of mixture = 525 mmHg