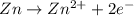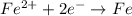Chemistry, 27.06.2020 09:01 wsdafvbhjkl
The hall reaction of an oxidation-reduction reaction shows that iron gains electrons. What does this electron gain mean for iron?
A
It is neutralized
B.
It is oxidized
C. It is reduced
D.
It has dissolved.
E
It has precipitated

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
You know the right answer?
The hall reaction of an oxidation-reduction reaction shows that iron gains electrons. What does this...
Questions

Geography, 05.09.2020 04:01

Chemistry, 05.09.2020 04:01





Mathematics, 05.09.2020 04:01

Computers and Technology, 05.09.2020 04:01




Mathematics, 05.09.2020 04:01

Mathematics, 05.09.2020 04:01

Mathematics, 05.09.2020 04:01


Social Studies, 05.09.2020 04:01



Mathematics, 05.09.2020 04:01