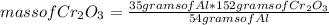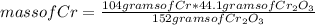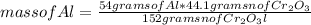Chemistry, 27.06.2020 15:01 deonnaturner68p7hz7y
How many grams of Cr can be produced by the reaction of 44.1 g of Cr2O3 with 35.0 g of Al according to the following chemical equation? How many grams of the excess react will remain once the reaction goes to completion. 2Al + Cr2O3 à Al2O3 + 2Cr

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3
You know the right answer?
How many grams of Cr can be produced by the reaction of 44.1 g of Cr2O3 with 35.0 g of Al according...
Questions

Mathematics, 10.11.2020 22:00



Mathematics, 10.11.2020 22:00


Social Studies, 10.11.2020 22:00


Arts, 10.11.2020 22:00


English, 10.11.2020 22:00

Mathematics, 10.11.2020 22:00

Business, 10.11.2020 22:00

Mathematics, 10.11.2020 22:00




Physics, 10.11.2020 22:00

Mathematics, 10.11.2020 22:00

English, 10.11.2020 22:00

Mathematics, 10.11.2020 22:00