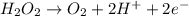Chemistry, 27.06.2020 18:01 nataliemakin7123
The concentration of hydrogen peroxide (H2O2) can be determined by titrating it with an acidified MnO4− solution. The following is an unbalanced equation.
MnO4− (aq) + H2O2 (aq) → O2 (g) + Mn2+ (aq)
(a) Balance the above redox reaction.
(b)Determine the concentration of H2O2 solution in molarity if 10.00 mL of this solution requires 20.00 mL of 1.5 M MnO4− for a complete reaction.

Answers: 2
Another question on Chemistry



Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1
You know the right answer?
The concentration of hydrogen peroxide (H2O2) can be determined by titrating it with an acidified Mn...
Questions

Mathematics, 05.12.2020 01:30

Mathematics, 05.12.2020 01:30

Arts, 05.12.2020 01:30





Biology, 05.12.2020 01:30



Mathematics, 05.12.2020 01:30



Mathematics, 05.12.2020 01:30





English, 05.12.2020 01:30

Mathematics, 05.12.2020 01:30