
Chemistry, 27.06.2020 02:01 sairaanwar67
Consider the following reversible reaction. CO(g)+2H2(g) CH3OH(g) What is the equilibrium constant expression for the given system?
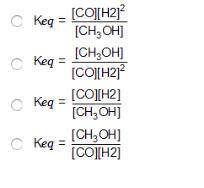
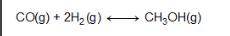

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
Consider the following reversible reaction. CO(g)+2H2(g) CH3OH(g) What is the equilibrium constant e...
Questions




Social Studies, 28.05.2020 18:59


Mathematics, 28.05.2020 18:59


English, 28.05.2020 18:59







Mathematics, 28.05.2020 18:59





Mathematics, 28.05.2020 18:59



