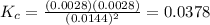Chemistry, 27.06.2020 15:01 cynthiafchs9203
Given the reaction: 2HF(g) H2(g) F2(g) If the initial concentration of HF is 0.025M and the equilibrium concentration of H2 is 0.0028M, then what is the equilibrium constant of the reaction

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
Given the reaction: 2HF(g) H2(g) F2(g) If the initial concentration of HF is 0.025M and the equilibr...
Questions

English, 23.10.2019 01:50



















![K_{c} =\frac{[H_{2}][F_{2}] }{[HF]^2}](/tpl/images/0695/8873/96c9f.png)