
The same reaction, with exactly the same amount of reactant, is conducted in a bomb calorimeter and in a coffee-cup calorimeter. In one measurement, qrxn=−13.3qrxn=−13.3 kJkJ in the other qrxn=−11.9qrxn=−11.9 kJkJ. Which value was obtained in the bomb calorimeter? (Assume that the reaction has a positive ΔVΔV in the coffee-cup calorimeter.)

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1

Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
You know the right answer?
The same reaction, with exactly the same amount of reactant, is conducted in a bomb calorimeter and...
Questions

Mathematics, 22.07.2019 10:30




Mathematics, 22.07.2019 10:30


English, 22.07.2019 10:30

Physics, 22.07.2019 10:30

Mathematics, 22.07.2019 10:30


English, 22.07.2019 10:30

Mathematics, 22.07.2019 10:30

History, 22.07.2019 10:30

Physics, 22.07.2019 10:30


Biology, 22.07.2019 10:30




Health, 22.07.2019 10:30

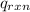 = -13.3 KJ belongs to the bomb calorimeter
= -13.3 KJ belongs to the bomb calorimeter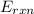 of the reaction, the work of the reaction
of the reaction, the work of the reaction 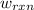 and the heat of the reaction
and the heat of the reaction 

