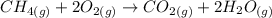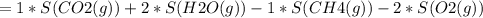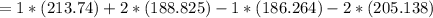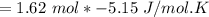Chemistry, 27.06.2020 18:01 destiny3200
Consider the reaction CH4(g) 2O2(g)CO2(g) 2H2O(g) Using standard thermodynamic data at 298K, calculate the entropy change for the surroundings when 1.62 moles of CH4(g) react at standard conditions.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
Consider the reaction CH4(g) 2O2(g)CO2(g) 2H2O(g) Using standard thermodynamic data at 298K, calcula...
Questions



Mathematics, 19.01.2020 11:31


World Languages, 19.01.2020 11:31




Mathematics, 19.01.2020 11:31


Spanish, 19.01.2020 11:31

Mathematics, 19.01.2020 11:31




Social Studies, 19.01.2020 11:31

History, 19.01.2020 11:31

Mathematics, 19.01.2020 11:31


Biology, 19.01.2020 11:31