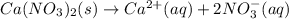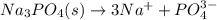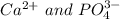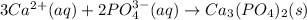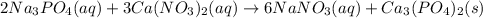Chemistry, 28.06.2020 07:01 woodsjnjoseph3
During lab, students mixed two solutions of soluble ions in a ceramic well to determine if a precipitate forms.
Write the dissolution reaction for the ionic solids below. (Use the lowest possible coefficients. Include states-of-matter under the given conditions in your answer.)
(a) Ca(NO3)2
(b) Na3PO4
The two solutions, when mixed, will have two cations and two anions.
(c) Based on your lab results, enter the cation and anion for which a precipitate will form. (Separate substances in a list with a comma.)
(d) Write the net precipitation reaction that occurs. (Use the lowest possible coefficients. Include states-of-matter under the given conditions in your answer.)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1
You know the right answer?
During lab, students mixed two solutions of soluble ions in a ceramic well to determine if a precipi...
Questions



Mathematics, 05.02.2021 16:40





History, 05.02.2021 16:40


Mathematics, 05.02.2021 16:40



Mathematics, 05.02.2021 16:40

Mathematics, 05.02.2021 16:40


Social Studies, 05.02.2021 16:40

Mathematics, 05.02.2021 16:40

Computers and Technology, 05.02.2021 16:40

Biology, 05.02.2021 16:40

Mathematics, 05.02.2021 16:40