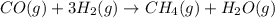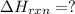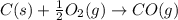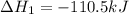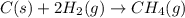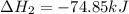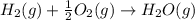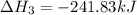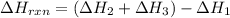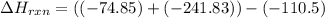Chemistry, 29.06.2020 03:01 priscillavaladez1112
Use Hess's law and the following equations to calculate the ΔHreaction for the reaction CO(g) + 3H2(g) CH4(g) + H2O(g). (Show your work.) (4 points) • C(s) + O2(g) CO(g) ΔH = –110.5 kJ • C(s) + 2H2(g) CH4(g) ΔH = –74.85 kJ • H2(g) + O2(g) H2O(g) ΔH = –241.83 kJ

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
Use Hess's law and the following equations to calculate the ΔHreaction for the reaction CO(g) + 3H2(...
Questions


Mathematics, 28.06.2021 22:50




English, 28.06.2021 22:50


Mathematics, 28.06.2021 22:50


Mathematics, 28.06.2021 22:50

Mathematics, 28.06.2021 22:50





Mathematics, 28.06.2021 22:50


Geography, 28.06.2021 22:50