
Chemistry, 29.06.2020 04:01 shawntawright1
The theoretical yield of zinc oxide in a reaction is 486 g. What is the percent yield if 399 g is produced?
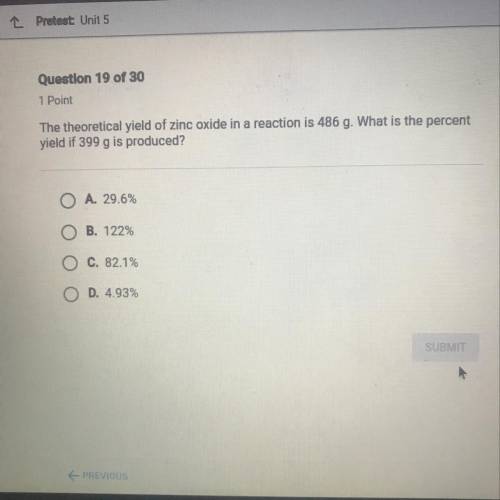

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1
You know the right answer?
The theoretical yield of zinc oxide in a reaction is 486 g. What is the percent
yield if 399 g is p...
Questions





Chemistry, 24.09.2019 01:10


Mathematics, 24.09.2019 01:10

Biology, 24.09.2019 01:10


English, 24.09.2019 01:10









Physics, 24.09.2019 01:10



