Chemistry, 28.06.2020 23:01 maustin5323
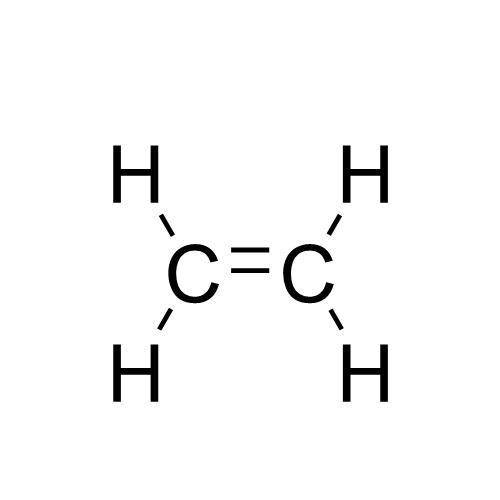
A compound with a molar mass of about 28 g/mol contains 85.7% carbon and 14.3% hydrogen by mass. Write the Lewis structure for a molecule of the compound.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1
You know the right answer?
A compound with a molar mass of about 28 g/mol contains 85.7% carbon and 14.3% hydrogen by mass. Wri...
Questions

Biology, 30.08.2019 14:50


Mathematics, 30.08.2019 14:50


History, 30.08.2019 14:50

Mathematics, 30.08.2019 14:50

Social Studies, 30.08.2019 14:50



Computers and Technology, 30.08.2019 14:50

World Languages, 30.08.2019 14:50

Mathematics, 30.08.2019 14:50

Biology, 30.08.2019 14:50



Spanish, 30.08.2019 14:50

Mathematics, 30.08.2019 14:50

World Languages, 30.08.2019 14:50

Mathematics, 30.08.2019 14:50