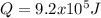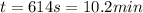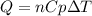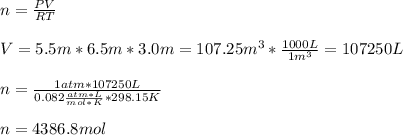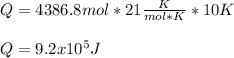The heat capacity of air is much smaller than that of water, and relatively modest amounts of heat are needed to change its temperature. This is one of the reasons why desert region, although very hot during the day, are bitterly cold at night. The heat capacity of air at room temperature and pressure is appoximately 21 J/K*mol. How much energy is required to raise the temperature of a room of dimensions 5.5m x 6.5m x 3.0m by 10 degrees Celsius? If losses are neglected, how long will it take a heater rated at 1.5 kW to achieve that increase given that 1 W = 1 J/s?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
The wilson chamber is used to study: direction, speed, and distance of radioactivity the intensity of radiation the appearance of individual atoms all of the above
Answers: 1



Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
The heat capacity of air is much smaller than that of water, and relatively modest amounts of heat a...
Questions

Mathematics, 25.11.2021 14:00

Mathematics, 25.11.2021 14:00




Mathematics, 25.11.2021 14:00

Mathematics, 25.11.2021 14:00


Mathematics, 25.11.2021 14:00


Mathematics, 25.11.2021 14:00

History, 25.11.2021 14:00





English, 25.11.2021 14:00

Computers and Technology, 25.11.2021 14:00

Social Studies, 25.11.2021 14:00

Physics, 25.11.2021 14:00