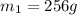Chemistry, 30.06.2020 18:01 6710000831
A silver block, initially at 56.1 ∘C, is submerged into 100.0 g of water at 24.0 ∘C, in an insulated container. The final temperature of the mixture upon reaching thermal equilibrium is 28.0∘C. What is the mass of the silver block?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1
You know the right answer?
A silver block, initially at 56.1 ∘C, is submerged into 100.0 g of water at 24.0 ∘C, in an insulated...
Questions






Engineering, 28.10.2020 17:10


English, 28.10.2020 17:10


Mathematics, 28.10.2020 17:10

Mathematics, 28.10.2020 17:10


English, 28.10.2020 17:10

Mathematics, 28.10.2020 17:10






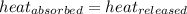
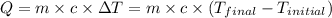
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0697/4196/09236.png) .................(1)
.................(1)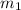 = mass of silver = ?
= mass of silver = ?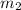 = mass of water = 100.0 g
= mass of water = 100.0 g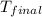 = final temperature =
= final temperature = 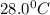
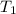 = temperature of silver =
= temperature of silver = 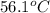
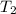 = temperature of water =
= temperature of water = 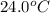
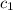 = specific heat of silver =
= specific heat of silver = 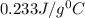
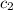 = specific heat of water=
= specific heat of water= 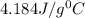
![-m_1\times 0.233\times (28.0-56.1)=[100.0\times 4.184\times (28.0-24.0)]](/tpl/images/0697/4196/c0833.png)