Chemistry, 01.07.2020 15:01 diegobebe503
At 700 K, CCl4 decomposes to carbon and chlorine. The Kp for the decomposition is 0.76. Find the starting pressure of CCl4 at this temperature that will produce a total pressure of 1.2 atm at equilibrium. P=?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1


Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2
You know the right answer?
At 700 K, CCl4 decomposes to carbon and chlorine. The Kp for the decomposition is 0.76. Find the sta...
Questions

Mathematics, 18.03.2021 01:20





English, 18.03.2021 01:20

English, 18.03.2021 01:20





Mathematics, 18.03.2021 01:20

Mathematics, 18.03.2021 01:20

Mathematics, 18.03.2021 01:20






English, 18.03.2021 01:20

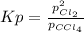
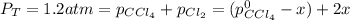
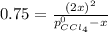
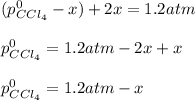
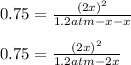
 we have to roots:
we have to roots: