pick the atom with

Chemistry, 30.06.2020 18:01 thutch1950oww9q0
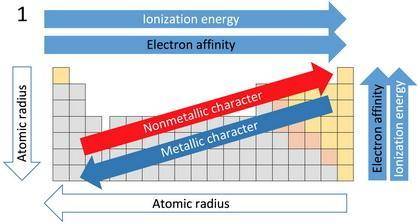
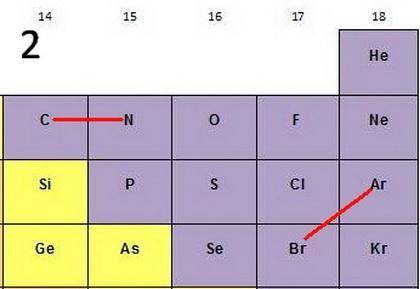
For each of the following pairs of elements
(1C and N2) (1Ar and Br2)
pick the atom with
a. more favorable (exothermic) electron affinity.
b. higher ionization energy.
c. larger size.
How do you even go about do this?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1


Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

You know the right answer?
For each of the following pairs of elements
(1C and N2) (1Ar and Br2)
pick the atom with
pick the atom with
Questions

English, 15.01.2021 01:30


Mathematics, 15.01.2021 01:30

Mathematics, 15.01.2021 01:30







Mathematics, 15.01.2021 01:30

Mathematics, 15.01.2021 01:30

Mathematics, 15.01.2021 01:30

Mathematics, 15.01.2021 01:30

History, 15.01.2021 01:30

Mathematics, 15.01.2021 01:30


History, 15.01.2021 01:30


Health, 15.01.2021 01:30