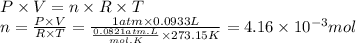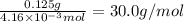Chemistry, 01.07.2020 18:01 salgado100400
Calculate the molar mass of a gaseous substance if 0.125 g of the gas occupies 93.3 mL at STP.
30.2 g/mol
30.4g/mol
30.6 g/mol
30.0 g/mol
None of the above

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1
You know the right answer?
Calculate the molar mass of a gaseous substance if 0.125 g of the gas occupies 93.3 mL at STP.
30.2...
Questions

Mathematics, 16.04.2020 19:40


Mathematics, 16.04.2020 19:40


History, 16.04.2020 19:40


Mathematics, 16.04.2020 19:40


English, 16.04.2020 19:40

Chemistry, 16.04.2020 19:40







History, 16.04.2020 19:41

Biology, 16.04.2020 19:41


History, 16.04.2020 19:41