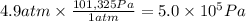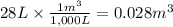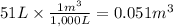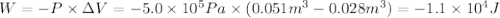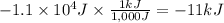Chemistry, 01.07.2020 23:01 thecoolgirl02
Calculate the work (kJ) done during a reaction in which the internal volume expands from 28 L to 51 L against an outside pressure of 4.9 atm. Calculate the work (kJ) done during a reaction in which the internal volume expands from 28 L to 51 L against an outside pressure of 4.9 atm. 11 kJ -11 kJ -39 kJ 39 kJ 0 kJ; No work is done.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1


Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2
You know the right answer?
Calculate the work (kJ) done during a reaction in which the internal volume expands from 28 L to 51...
Questions



Mathematics, 29.09.2021 08:30





Health, 29.09.2021 08:30


Biology, 29.09.2021 08:40

Mathematics, 29.09.2021 08:40

History, 29.09.2021 08:50

Mathematics, 29.09.2021 08:50


Mathematics, 29.09.2021 08:50

History, 29.09.2021 08:50