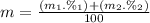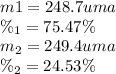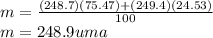Chemistry, 02.10.2019 14:50 Jsquad8879
Calculate the average atomic mas of an unknown element that has two naturally-occurring isotopes.
the first isotope occurs 75.47% of the time and has a mass of 248.7 a. m.u.
the second isotope occurs 24.53% of the time and has a mass of 249.4 a. m.u.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1
You know the right answer?
Calculate the average atomic mas of an unknown element that has two naturally-occurring isotopes.
Questions


Mathematics, 07.12.2020 14:00



Mathematics, 07.12.2020 14:00

English, 07.12.2020 14:00



English, 07.12.2020 14:00

Mathematics, 07.12.2020 14:00







Mathematics, 07.12.2020 14:00