
Chemistry, 02.07.2020 09:01 luvpeaceandsocc3678
Consider the chemical reaction below. Zn(s) + 2H+(aq) -> Zn2+(aq) + H2(g). Which half reaction correctly represents reduction for this equation?
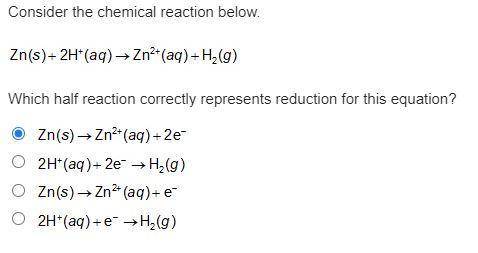

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 21.06.2019 20:10
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

You know the right answer?
Consider the chemical reaction below. Zn(s) + 2H+(aq) -> Zn2+(aq) + H2(g). Which half reaction co...
Questions










Mathematics, 23.07.2021 01:40

Biology, 23.07.2021 01:40


Medicine, 23.07.2021 01:40









