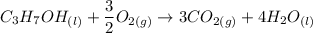The combustion of 1.685 g of propanol (C3H7OH) increases the temperature of a bomb calorimeter from 298.00 K to 302.16 K. The heat capacity of the bomb calorimeter is 13.60 kJ/K . Determine ΔH for the combustion of propanol to carbon dioxide gas and liquid water. g

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Look at the reaction below: ca(hco3)2 --> caco3 + co2 + h2o first, balance the reaction. once balanced, use dimensional analysis or another method to find out how many moles of carbon dioxide will be produced if we start with 16.5 moles of calcium bicarbonate (calcium hydrogen carbonate). = mol of co2 number needs to be reported to three significant figures.
Answers: 1

Chemistry, 21.06.2019 20:30
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2


Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1
You know the right answer?
The combustion of 1.685 g of propanol (C3H7OH) increases the temperature of a bomb calorimeter from...
Questions

English, 29.01.2020 08:46


Social Studies, 29.01.2020 08:46



English, 29.01.2020 08:46

English, 29.01.2020 08:46




Mathematics, 29.01.2020 08:46

Biology, 29.01.2020 08:46


History, 29.01.2020 08:46


History, 29.01.2020 08:46

Mathematics, 29.01.2020 08:46


Biology, 29.01.2020 08:46