
Chemistry, 02.07.2020 17:01 tejasheree
The cytochromes are heme‑containing proteins that function as electron carriers in the mitochondria. Calculate the difference in the reduction potential (ΔE∘′) and the change in the standard free energy (ΔG∘′) when the electron flow is from the carrier with the lower reduction potential to the higher. cytochrome c1 (Fe3+)+e−↽−−⇀cytochrome c1 (Fe2+)E∘′=0.22 V cytochrome c (Fe3+)+e−↽−−⇀cytochrome c (Fe2+)E∘′=0.254 V Calculate ΔE∘′ and ΔG∘′ .

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1



Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2
You know the right answer?
The cytochromes are heme‑containing proteins that function as electron carriers in the mitochondria....
Questions

Mathematics, 24.10.2020 05:20






Mathematics, 24.10.2020 05:20

Arts, 24.10.2020 05:20


Computers and Technology, 24.10.2020 05:20

Mathematics, 24.10.2020 05:20


Mathematics, 24.10.2020 05:20

History, 24.10.2020 05:20

Mathematics, 24.10.2020 05:20


Mathematics, 24.10.2020 05:20


Mathematics, 24.10.2020 05:20



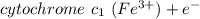 ⇔
⇔
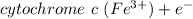 ⇔
⇔







