Chemistry, 03.07.2020 22:01 bellbradshaw16
In the first 15.0 s of the reaction, 1.7×10−2 mol of O2 is produced in a reaction vessel with a volume of 0.440 L . What is the average rate of the reaction over this time interval?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 23.06.2019 10:40
Question 17 hydrogen is manufactured on an industrial scale by this sequence of reactions: +ch4gh2og ⇌ +cog3h2g k1 +cogh2og ⇌ +co2gh2g k2 the net reaction is: +ch4g2h2og ⇌ +co2g4h2g k write an equation that gives the overall equilibrium constant k in terms of the equilibrium constants k1 and k2. if you need to include any physical constants, be sure you use their standard symbols, which you'll find in the aleks calculator.
Answers: 2

Chemistry, 23.06.2019 15:30
Among these processes, which is the slowest chemical reaction? a. digesting food b. boiling an egg c. tarnishing of silver d. melting of a glacier
Answers: 2

Chemistry, 23.06.2019 16:50
How can a scientist assess whether a pure niobium (nb) sample is responsible for contaminating the lab with radioactivity? test the niobium sample to see whether it now contains other elements.test the niobium sample for the presence of niobium oxide compounds.heat the niobium, and see if the level of radioactivity in the lab increases.place the niobium under pressure, and see if the level of radioactivity in the lab increases.
Answers: 3
You know the right answer?
In the first 15.0 s of the reaction, 1.7×10−2 mol of O2 is produced in a reaction vessel with a volu...
Questions







Business, 02.12.2019 01:31

History, 02.12.2019 01:31











History, 02.12.2019 01:31

English, 02.12.2019 01:31

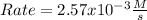
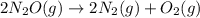
![[O_2]=\frac{0.017mol}{0.440L}=0.0386M](/tpl/images/0700/8490/f92ae.png)