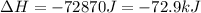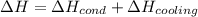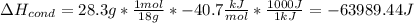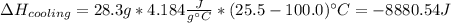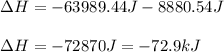Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1


Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2
You know the right answer?
How much total energy is released to cool 28.3 g of steam (water vapor) at 100.0°C to liquid water a...
Questions

History, 08.12.2020 02:50



Arts, 08.12.2020 02:50


Arts, 08.12.2020 02:50


Physics, 08.12.2020 02:50

Physics, 08.12.2020 02:50



English, 08.12.2020 02:50

Mathematics, 08.12.2020 02:50



Mathematics, 08.12.2020 02:50

History, 08.12.2020 02:50

Mathematics, 08.12.2020 02:50