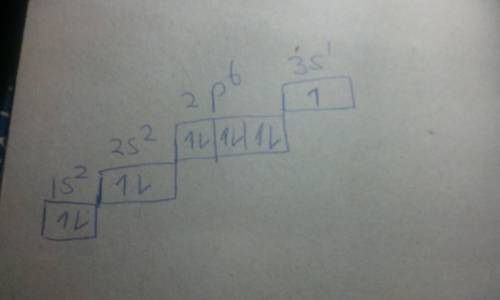
Chemistry, 04.07.2020 01:01 GreenHerbz206
The excited state of an atom is one in which an electron is in a higher energy level (or sublevel) than it is in its ground state (the "normal" electron configuration). The energy level to which the electron transitions must be an allowable energy level (for example an electron cannot transition from a ls to a lp sublevel because the lp sublevel does not exist). An excited state for Na, above its ground state, is [Ne]3p'. When the electron transitions back to the ground state, it emits the yellow-orange light characteristic of Na. The energy of this photon of light is equal to the energy difference between the 3p and the orbital in which the electron is located in the ground state.
a) Draw the ground state electron configuration and orbital filling diagram for Na in its ground state.
b) Another excited state for Na is [Ne]4p'. Which of the following could describe the radiation emitted by the Na atom as it transitions back to the ground state: UV light, the same yellow orange light as described above, red light, or infrared light? Explain.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1

Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1
You know the right answer?
The excited state of an atom is one in which an electron is in a higher energy level (or sublevel) t...
Questions

Mathematics, 21.10.2020 22:01



Mathematics, 21.10.2020 22:01




Mathematics, 21.10.2020 22:01





Mathematics, 21.10.2020 22:01

History, 21.10.2020 22:01




English, 21.10.2020 22:01