Chemistry, 04.07.2020 01:01 Chandler1Gaming
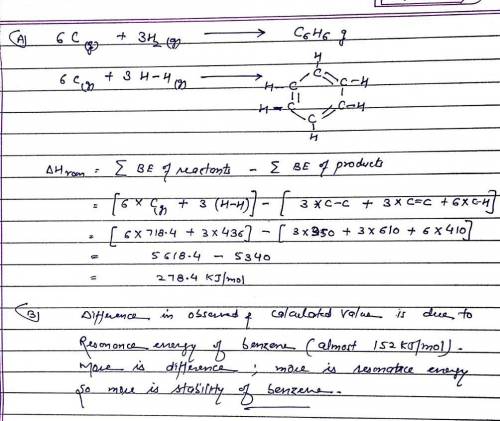
A. Use average bond energies together with the standard enthalpy of formation of C(g) (718.4 kJ/mol ) to estimate the standard enthalpy of formation of gaseous benzene, C6H6(g). (Remember that average bond energies apply to the gas phase only.) B. Compare the value you obtain using average bond energies to the actual standard enthalpy of formation of gaseous benzene, 82.9 kJ/mol. What does the difference between these two values tell you about the stability of benzene?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1


Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
A. Use average bond energies together with the standard enthalpy of formation of C(g) (718.4 kJ/mol...
Questions

Biology, 27.10.2020 16:00

Mathematics, 27.10.2020 16:00





Mathematics, 27.10.2020 16:00

English, 27.10.2020 16:00

Biology, 27.10.2020 16:00

Mathematics, 27.10.2020 16:00


Mathematics, 27.10.2020 16:00

Mathematics, 27.10.2020 16:00




Mathematics, 27.10.2020 16:00

Mathematics, 27.10.2020 16:00