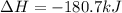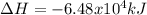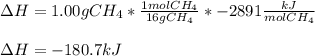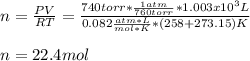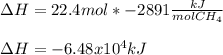Consider the following reaction:
CH4 +2O2 → CO2 + 2H2O. ΔH= -2891 kJ
Calculate the enth...

Chemistry, 04.07.2020 02:01 khikhi1705
Consider the following reaction:
CH4 +2O2 → CO2 + 2H2O. ΔH= -2891 kJ
Calculate the enthalpy change for each of the following cases:
a. 1.00 g methane is burned in excess oxygen.
b. 1.00 3 10^3 L methane gas at 740. torr and 258°C are burned in excess oxygen.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
You know the right answer?
Questions



Biology, 27.04.2021 03:20


Mathematics, 27.04.2021 03:20

Geography, 27.04.2021 03:20


Mathematics, 27.04.2021 03:20




Computers and Technology, 27.04.2021 03:20

Chemistry, 27.04.2021 03:20


Social Studies, 27.04.2021 03:20

Chemistry, 27.04.2021 03:20

Advanced Placement (AP), 27.04.2021 03:20