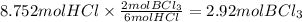Question 1
1 pts
2B+6HCI --
| --> 2BCl3 + 3H2
How many moles of boron chloride...


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1


Chemistry, 23.06.2019 11:40
Which of the following is true for a reliable scientific source? it cites logic. it cites opinions. it cites valid data. it cites common sense.
Answers: 2
You know the right answer?
Questions


Mathematics, 30.05.2021 22:50

Chemistry, 30.05.2021 22:50




Mathematics, 30.05.2021 22:50



Social Studies, 30.05.2021 22:50

Physics, 30.05.2021 22:50

Physics, 30.05.2021 22:50


Social Studies, 30.05.2021 22:50



Arts, 30.05.2021 22:50

Mathematics, 30.05.2021 22:50

English, 30.05.2021 22:50

Mathematics, 30.05.2021 22:50