A proposed mechanism for the reaction of NO2 and CO is
Step 1: Slow, endothermic:
2 NO2 (g) → NO (g) + NO3 (g)
Step 2: Fast, exothermic:
NO3 (g) + CO (g) → NO2 (g) + CO2 (g)
Overall reaction, exothermic:
NO2 (g) + CO (g) → NO (g) + CO2 (g)
a. Identify each of the following as a reactant, product, or intermediate:
1. NO2(g)
2. CO(g)
3. NO3(g)
4. CO2(g)
5. NO(g)
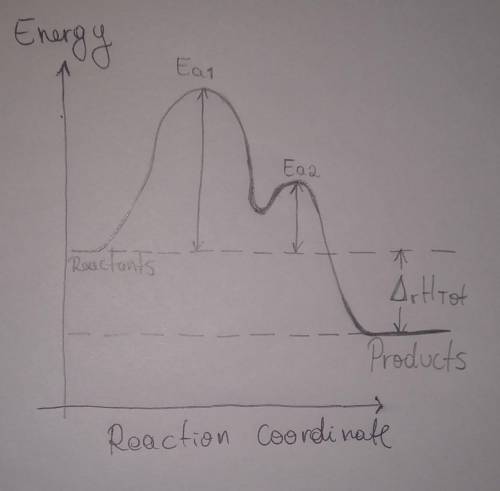
b. Draw a reaction coordinate diagram for this reaction. Indicate on this drawing the activation energy for each step and the overall enthalpy change.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1


Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

You know the right answer?
A proposed mechanism for the reaction of NO2 and CO is
Step 1: Slow, endothermic:
2 NO2 (g)...
2 NO2 (g)...
Questions

Mathematics, 26.03.2021 04:30




Mathematics, 26.03.2021 04:30


Biology, 26.03.2021 04:30

Mathematics, 26.03.2021 04:30

Mathematics, 26.03.2021 04:30

Advanced Placement (AP), 26.03.2021 04:30



History, 26.03.2021 04:30

Mathematics, 26.03.2021 04:30





Mathematics, 26.03.2021 04:30

Mathematics, 26.03.2021 04:30