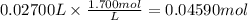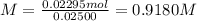Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
A 25.00 mL solution of sulfuric acid (H2SO4) is titrated to phenolphthalein end point with 27.00 mL...
Questions

Mathematics, 12.11.2020 03:30

Biology, 12.11.2020 03:30


English, 12.11.2020 03:30

Computers and Technology, 12.11.2020 03:30


History, 12.11.2020 03:30


Biology, 12.11.2020 03:30

Mathematics, 12.11.2020 03:30

Physics, 12.11.2020 03:30


Physics, 12.11.2020 03:30

Mathematics, 12.11.2020 03:30




Spanish, 12.11.2020 03:30