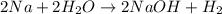How many of the following are true concerning balanced chemical equations? I. The number of molecules is conserved. II. The coefficients for the reactants tell you how much of each reactant you are given. III. Atoms are neither created nor destroyed. IV. The coefficients indicate the mass ratios of the substances used. V. The sum of the coefficients on the reactant side equals the sum of the coefficients on the product side.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

You know the right answer?
How many of the following are true concerning balanced chemical equations? I. The number of molecule...
Questions


Arts, 01.09.2020 19:01




Mathematics, 01.09.2020 19:01



Mathematics, 01.09.2020 19:01



Chemistry, 01.09.2020 19:01


Computers and Technology, 01.09.2020 19:01



Mathematics, 01.09.2020 19:01

Mathematics, 01.09.2020 19:01