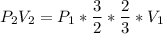Chemistry, 05.07.2020 14:01 jakobrobinette
Consider this reaction at equilibrium at a total pressure P1: 2SO2(g) + O2(g) → 2SO3(g) Suppose the volume of this system is compressed to one-half its initial volume and then equilibrium is reestablished. The new equilibrium total pressure will be:

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2

Chemistry, 23.06.2019 08:30
This has nothing to do with school. i wrote a poem to my crush, who i'm asking out soon. tell me if it's cheesy, or cute. "roses are red, violets are blue no love story sounds right if it doesn't include you. dance with me all night, gaze into my eyes i'll hand you my heart, as well as my pride. when i hear your name, my heart goes insane. your all that i want, all that i need promise me you'll stay with me. here it is the final line, jasmine hill will you be mine? " i'm also going to buy her flowers, teddy bear and some food lol. written by me, bre (:
Answers: 2

Chemistry, 23.06.2019 09:20
1) a. water molecule breaks up into hydrogen and oxygen on passing electricity. does this involve breaking intermolecular or intramolecular forces of attraction. explain b. on boiling water changes to water vapor. does this involve breaking intermolecular or intramolecular forces of attraction. explain methanol evaporates faster than water. contrast the intermolecular forces and the vapor pressures of methanol and water?
Answers: 2

Chemistry, 23.06.2019 09:30
My plate recommends that of your nutritional intake comes from fruits and vegetables. a. 30% b. 50% c. 20% d. 40%
Answers: 2
You know the right answer?
Consider this reaction at equilibrium at a total pressure P1: 2SO2(g) + O2(g) → 2SO3(g) Suppose th...
Questions


Geography, 27.12.2019 02:31

Mathematics, 27.12.2019 02:31

History, 27.12.2019 02:31

Mathematics, 27.12.2019 02:31

History, 27.12.2019 02:31

Computers and Technology, 27.12.2019 02:31