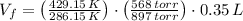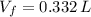Chemistry, 05.07.2020 14:01 gameranonymous266
A sample of 0.35 L of argon gas (at a temperature of 13 oC and a pressure of 568 torr) is heated to 156 oC and a new pressure of 897 torr. Calculate the new volume of the gas.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 23.06.2019 07:30
How do you interpret a chromagram for what mixtures contain?
Answers: 1

Chemistry, 23.06.2019 09:30
Sheela and her brother hari were sitting in the living room, watching tv. suddenly hari said that he thinks something is burning in the other room. how did he get the burning smell?
Answers: 3

Chemistry, 23.06.2019 18:40
Explain how electricity can be conducted by acids and bases
Answers: 1
You know the right answer?
A sample of 0.35 L of argon gas (at a temperature of 13 oC and a pressure of 568 torr) is heated to...
Questions

Mathematics, 22.06.2021 02:30

English, 22.06.2021 02:30

Mathematics, 22.06.2021 02:30


Mathematics, 22.06.2021 02:30



Advanced Placement (AP), 22.06.2021 02:30




Mathematics, 22.06.2021 02:30



Physics, 22.06.2021 02:30

Biology, 22.06.2021 02:30


English, 22.06.2021 02:30

English, 22.06.2021 02:30

English, 22.06.2021 02:30

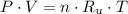
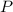 - Pressure, measured in torr.
- Pressure, measured in torr.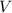 - Volume, measured in liters.
- Volume, measured in liters.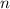 - Molar quantity, measured in moles.
- Molar quantity, measured in moles. 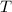 - Temperature, measured in kelvins.
- Temperature, measured in kelvins.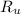 - Ideal gas constant, measured in
- Ideal gas constant, measured in 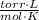 .
.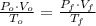
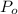 ,
, 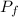 - Initial and final pressures, measured in torr.
- Initial and final pressures, measured in torr.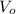 ,
, 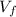 - Initial and final volumes, measured in liters.
- Initial and final volumes, measured in liters.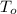 ,
, 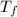 - Initial and final temperatures, measured in kelvins.
- Initial and final temperatures, measured in kelvins. 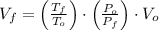
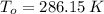 ,
, 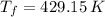 ,
, 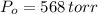 ,
, 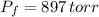 and
and 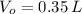 , the new volume of the gas is:
, the new volume of the gas is: