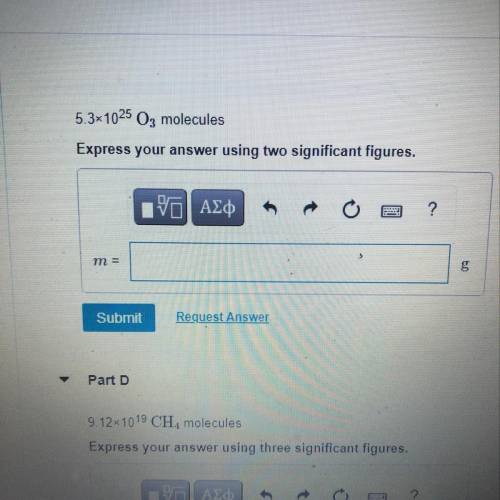
Calculate the mass in grams of each sample.
4.88x10^20 H2O2 molecules
...


Answers: 3
Another question on Chemistry


Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2

Chemistry, 23.06.2019 04:00
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1

Chemistry, 23.06.2019 05:00
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2
You know the right answer?
Questions



Mathematics, 05.02.2020 03:02

Mathematics, 05.02.2020 03:02



Mathematics, 05.02.2020 03:02

Mathematics, 05.02.2020 03:02


Mathematics, 05.02.2020 03:02

Mathematics, 05.02.2020 03:02

Mathematics, 05.02.2020 03:02





Mathematics, 05.02.2020 03:02

Mathematics, 05.02.2020 03:02

Health, 05.02.2020 03:02