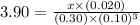Carbon monoxide (CO) reacts with hydrogen (H2) to form methane (CH4) and water (H20).
CO(g) + 3H2(g) + CH4(g)+H20(9)
The reaction is at equilibrium at 1,000 K. The equilibrium constant of the reaction is 3.90. At equilibrium, the concentrations are as
follows.
[CO] = 0.30 M
[H2] = 0.10 M
[H20] = 0.020 M
What is the equilibrium concentration of CH, expressed in scientific notation?
0.0059
5.9 x 10-2
0.059
5.9 x 102

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2


Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1
You know the right answer?
Carbon monoxide (CO) reacts with hydrogen (H2) to form methane (CH4) and water (H20).
CO(g) + 3H2(g...
Questions

Mathematics, 19.01.2021 20:10

Mathematics, 19.01.2021 20:10

Mathematics, 19.01.2021 20:10


Mathematics, 19.01.2021 20:10

History, 19.01.2021 20:10






Mathematics, 19.01.2021 20:10

Geography, 19.01.2021 20:10


Social Studies, 19.01.2021 20:10





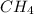 , expressed in scientific notation is
, expressed in scientific notation is 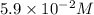
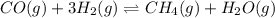
![K_c=\frac{[CH_4]\times [H_2O]}{[CO]\times [H_2]^3}](/tpl/images/0702/2496/1cdb8.png)