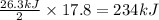Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1
You know the right answer?
The reduction of iron(III) oxide to iron metal is an endothermic process: Fe2O3(s) + 2 CO(g) → 2 Fe(...
Questions

Mathematics, 18.12.2020 19:40




Mathematics, 18.12.2020 19:40

Health, 18.12.2020 19:40


Mathematics, 18.12.2020 19:40

Mathematics, 18.12.2020 19:40




Biology, 18.12.2020 19:40

Mathematics, 18.12.2020 19:40

Mathematics, 18.12.2020 19:40

Spanish, 18.12.2020 19:40

History, 18.12.2020 19:40

Mathematics, 18.12.2020 19:40


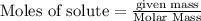
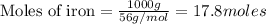 (1.00kg=1000g)
(1.00kg=1000g)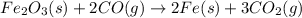
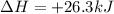
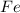 is produced = 26.3 kJ
is produced = 26.3 kJ