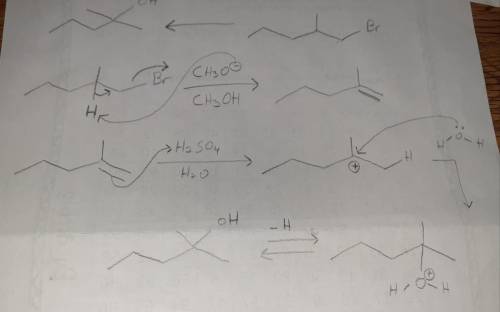
Design a Synthesis of 2-methyl-2-pentanol from 1-bromo-2-methylpentane Part 1: Choose the best option for the immediate precursor to the target molecule. An alkene that has greater substitution on one end of the double bond permits you to control the regiochemistry, placing the OH group selectively on one of the two double bond carbons. This alkene is the best choice, considering the starting material that you have available. Part 2: Choose the best option for the precursor needed to make the alkene. Alkenes can be made in good yield by elimination of HBr from an alkyl halide. Whenever possible, use an alkyl halide that can only make a single alkene by HBr elimination. Part 3: Here is an overview of your retrosynthesis (there is no work, this is just a recap). Part 4 out of 6 Choose the most appropriate reagent(s) for the first step of the synthesis.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2

Chemistry, 21.06.2019 20:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 21.06.2019 22:30
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

You know the right answer?
Design a Synthesis of 2-methyl-2-pentanol from 1-bromo-2-methylpentane Part 1: Choose the best optio...
Questions

History, 22.06.2020 20:57



Spanish, 22.06.2020 20:57


Health, 22.06.2020 20:57



Mathematics, 22.06.2020 20:57


Mathematics, 22.06.2020 20:57






Health, 22.06.2020 20:57

Computers and Technology, 22.06.2020 20:57

History, 22.06.2020 20:57

Mathematics, 22.06.2020 20:57