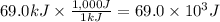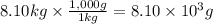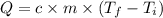Chemistry, 08.07.2020 02:01 sanchezp0821
A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 8.10kg of water at 33.9 degrees celsius . During the reaction 69.0kJ of heat flows out of the bath and into the flask. Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18J*g*K.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3
You know the right answer?
A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 8....
Questions


Biology, 14.07.2019 01:00

Biology, 14.07.2019 01:00

Mathematics, 14.07.2019 01:00


Mathematics, 14.07.2019 01:00

Computers and Technology, 14.07.2019 01:00


Health, 14.07.2019 01:00




Geography, 14.07.2019 01:00

Geography, 14.07.2019 01:00


Geography, 14.07.2019 01:00

Geography, 14.07.2019 01:00

Geography, 14.07.2019 01:00

Geography, 14.07.2019 01:00