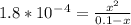Chemistry, 07.07.2020 19:01 rosie20052019
Calculate the pH for the following 1.0M weak acid solutions:a. HCOOH Ka = 1.8 x 10-4 [

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3
You know the right answer?
Calculate the pH for the following 1.0M weak acid solutions:a. HCOOH Ka = 1.8 x 10-4 [...
Questions

Mathematics, 24.05.2021 16:20


Chemistry, 24.05.2021 16:20





Mathematics, 24.05.2021 16:20

Mathematics, 24.05.2021 16:20

Mathematics, 24.05.2021 16:20


Mathematics, 24.05.2021 16:20


Mathematics, 24.05.2021 16:30

Chemistry, 24.05.2021 16:30

Mathematics, 24.05.2021 16:30

Physics, 24.05.2021 16:30

Mathematics, 24.05.2021 16:30


French, 24.05.2021 16:30

![K_{a} =\frac{product}{reactant}=\frac{[H^+][HCOO^-]}{[HCOOH]}](/tpl/images/0702/5573/bdea7.png) .
.