
Chemistry, 07.07.2020 19:01 JayLiz1737
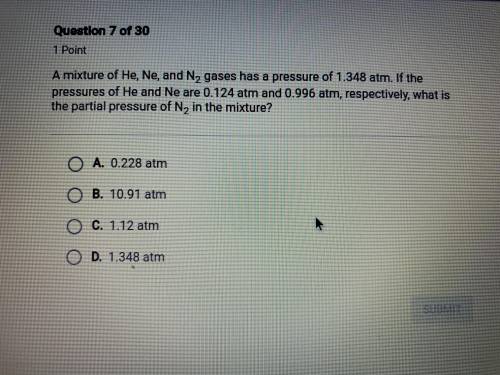
hurry please! a mixture of He, Ne, and N2 gases has a pressure of 1.348 atm. if the pressures of He and Ne are 0.124 atm and 0.996 atm, respectively, what is the partial pressure of N2 in the mixture?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1


You know the right answer?
hurry please! a mixture of He, Ne, and N2 gases has a pressure of 1.348 atm. if the pressures of He...
Questions



Mathematics, 12.06.2020 15:57





Geography, 12.06.2020 15:57








English, 12.06.2020 15:57

Mathematics, 12.06.2020 15:57

English, 12.06.2020 15:57

Mathematics, 12.06.2020 15:57

Mathematics, 12.06.2020 15:57



