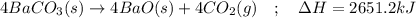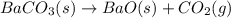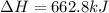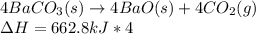Chemistry, 09.07.2020 01:01 Paytonsmommy09
Given the thermochemical expression
BaO (s) + CO2 (g) =
BaCO3(s)
AH° = -662.8 kJ
Write the thermochemical expression for the production of 4 mol CO2 by decomposition of solid
barium carbonate.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1
You know the right answer?
Given the thermochemical expression
BaO (s) + CO2 (g) =
BaCO3(s)
AH° = -662.8 kJ
...
BaCO3(s)
AH° = -662.8 kJ
...
Questions

Mathematics, 18.03.2021 23:10



English, 18.03.2021 23:10

Computers and Technology, 18.03.2021 23:10


Mathematics, 18.03.2021 23:10

Biology, 18.03.2021 23:10

Physics, 18.03.2021 23:10

Mathematics, 18.03.2021 23:10



Arts, 18.03.2021 23:10



Mathematics, 18.03.2021 23:10


English, 18.03.2021 23:10

Mathematics, 18.03.2021 23:10

Mathematics, 18.03.2021 23:10