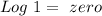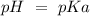Chemistry, 09.07.2020 01:01 jybuccaneers2022
50 mL of 0.1 M acetic acid is mixed with 50 mL of 0.1 M sodium acetate (the conjugate base). The Ka of acetic acid is approximately 1. 74 X 10 -5. What is the pH of the resulting solution?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1
You know the right answer?
50 mL of 0.1 M acetic acid is mixed with 50 mL of 0.1 M sodium acetate (the conjugate base). The Ka...
Questions




Mathematics, 02.11.2020 08:00

English, 02.11.2020 08:00

Mathematics, 02.11.2020 08:00

Mathematics, 02.11.2020 08:00

Physics, 02.11.2020 08:00


Biology, 02.11.2020 08:00




Mathematics, 02.11.2020 08:00

Health, 02.11.2020 08:00


Chemistry, 02.11.2020 08:00

Mathematics, 02.11.2020 08:00

Mathematics, 02.11.2020 08:00

Physics, 02.11.2020 08:00

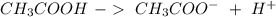
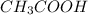 ) and a base (
) and a base (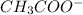 ). Therefore we can write the henderson-hasselbach reaction:
). Therefore we can write the henderson-hasselbach reaction:![pH~=~pKa+Log\frac{[CH_3COO^-]}{[CH_3COOH]}](/tpl/images/0703/3490/99062.png)
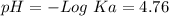
![[CH_3COO^-]=[CH_3COOH]](/tpl/images/0703/3490/ee54c.png)
![\frac{[CH_3COO^-]}{[CH_3COOH]}~=~1](/tpl/images/0703/3490/6e489.png)